View the latest BOT/BAL presentation from ESMO-GI
Our mission is to harness the power of the immune system to bring therapies with curative intent to individuals living with cancer
Latest News
Agenus’ BOT/BAL Achieves 42% Two-Year Survival in Refractory MSS CRC, Advances Toward Registration with FDA Alignment on Phase 3
Recent Publications
Learn more about our latest:
- Medical Congress Presentations
- Conference Posters
- Published Manuscripts
Agenus Medical Information
Here to answer your questions.
- General Medical Information
- Contact Request
- Investigator Sponsored Clinical Trials
- Access to Investigational Medicines
Spotlight on botensilimab
Agenus’ next-generation CTLA-4 antibody
Botensilimab was designed to:
- Extend the curative benefits of I-O to cold tumors that do not respond to approved immunotherapies. This is achieved by optimizing T cell interactions to drive increased potency.
- Expand clinical benefit in hot tumors such as melanoma, where CTLA-4 is approved, but reported results show clinical benefit in only a third of patients and long term survival in less than a quarter of patients.
- Improve tolerability by reducing or eliminating side effects that cause treatment discontinuation.
Innovation in immunotherapy
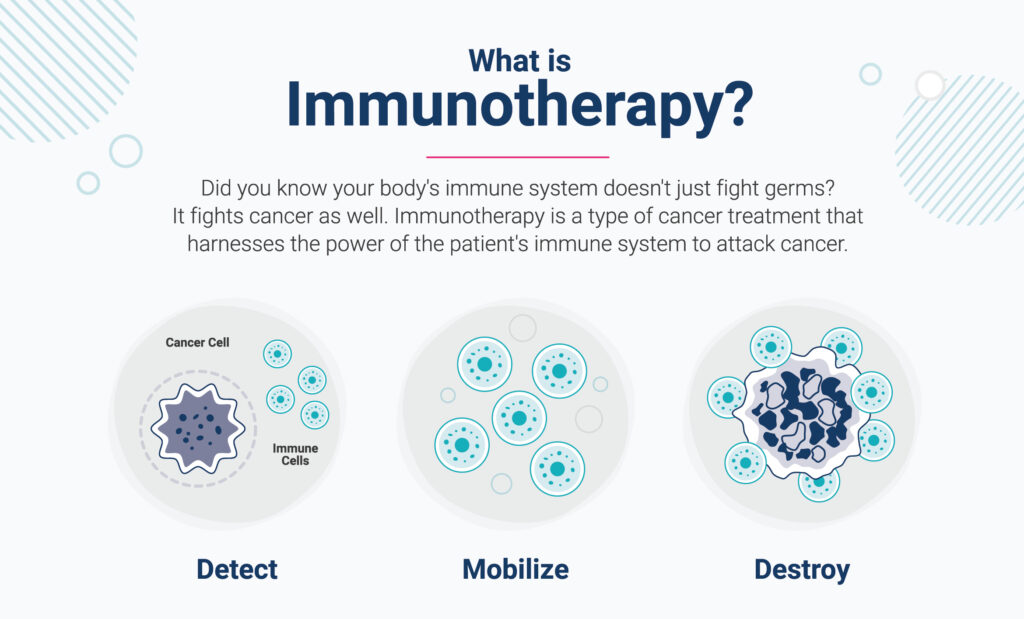
What is immunotherapy for cancer?
Today, we can create drugs that empower the immune system to do its job – cure disease.
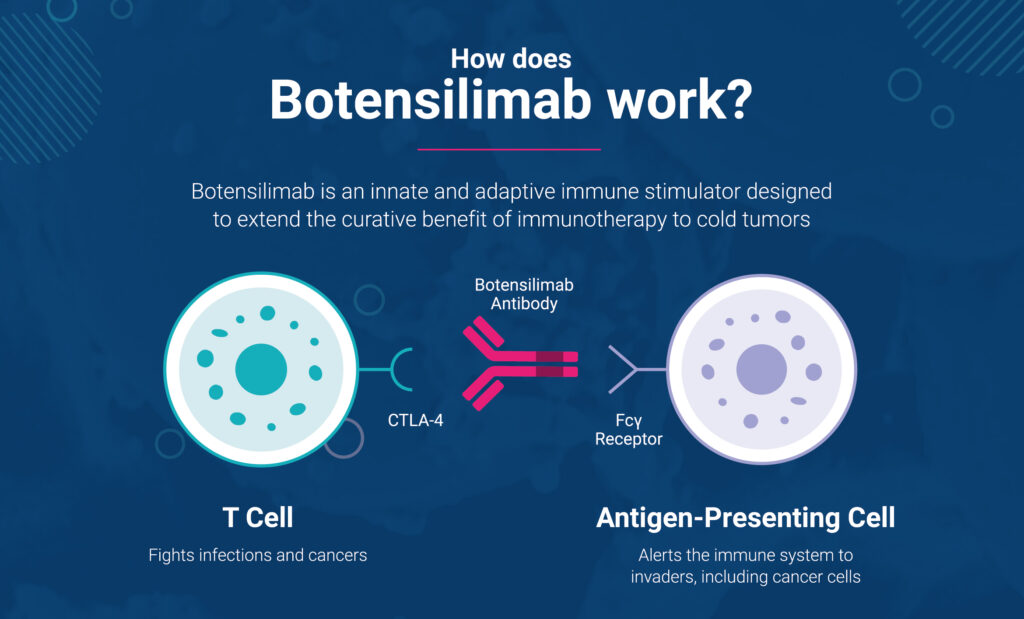
How does botensilimab work?
By modifying the Fc “tail” of the antibody, our scientists discovered that its efficacy and tolerability can be significantly enhanced.
By the numbers
Agenus discovers, manufactures, and develops immuno-oncology (I-O) therapeutics. Our vision is to expand the patient population benefiting from I-O through combination approaches that leverage a broad repertoire of antibody therapeutics, adoptive cell therapies (through subsidiary MiNK Therapeutics), adjuvants and vaccines (through subsidiary SaponiQx).
Acceleration through partnerships





Who we are
We have brought together top talent and expertise to advance our mission, building a team of employees across our offices in the US and Europe.