Our mission is to harness the power of the immune system to bring curative therapies to cancer patients
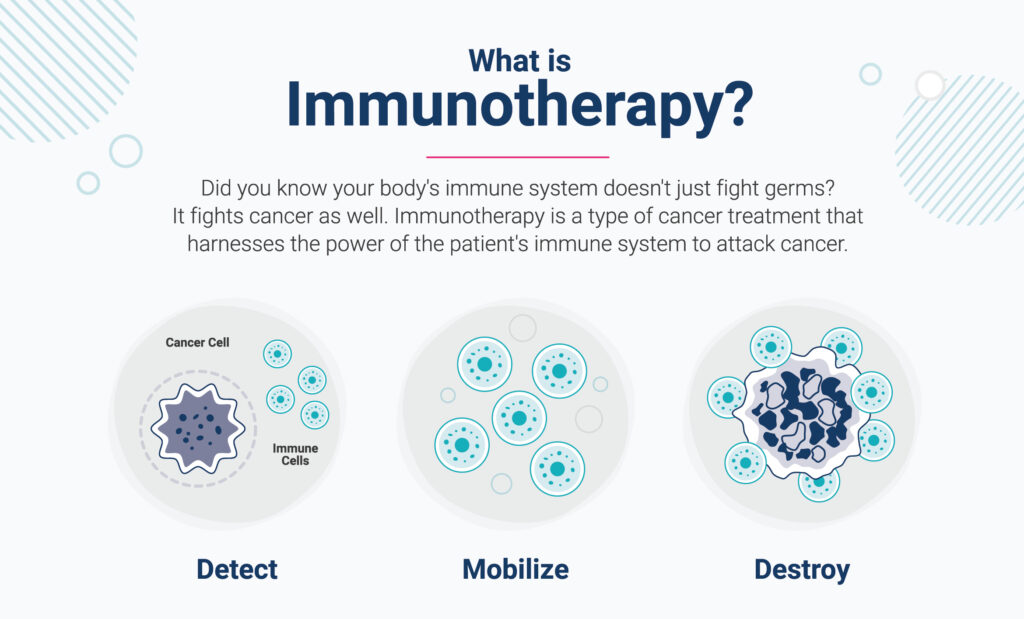
Today, we can create drugs that empower the immune system to do its job – cure disease.
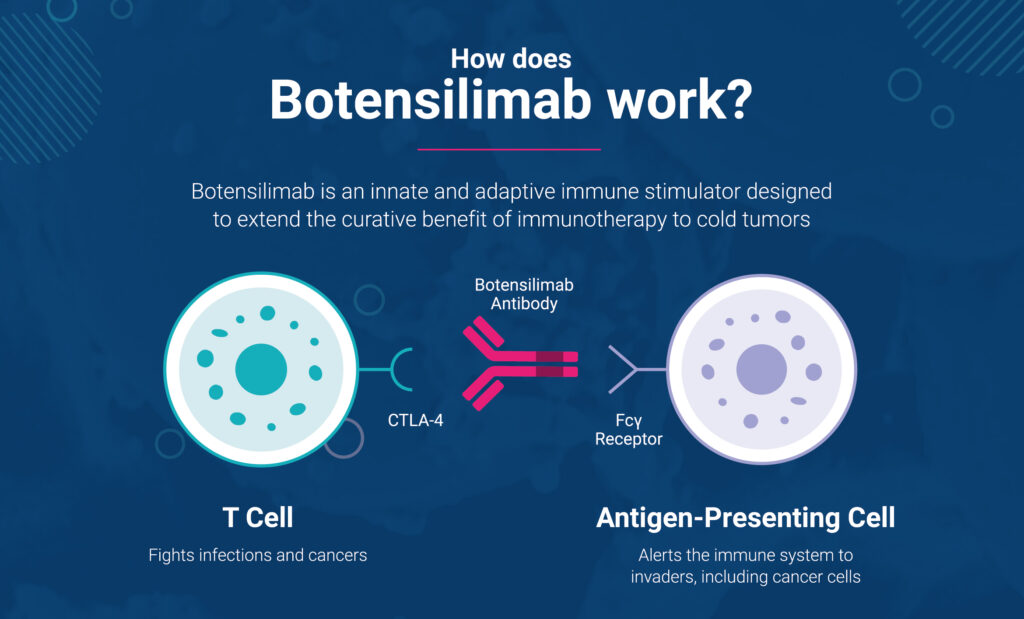
How does botensilimab work?
By modifying the Fc “tail” of the antibody, our scientists discovered that its efficacy and tolerability can be significantly enhanced.
Agenus discovers, manufactures, and develops immuno-oncology (I-O) therapeutics. Our vision is to expand the patient population benefiting from I-O through combination approaches that leverage a broad repertoire of antibody therapeutics, adoptive cell therapies (through subsidiary MiNK Therapeutics), adjuvants and vaccines (through subsidiary SaponiQx).





We have brought together top talent and expertise to advance our mission, building a team of employees across our offices in the US and Europe.
Agenus Inc.
3 Forbes Road
Lexington, MA 02421-7305
T: +1 781.674.4400 | F: +1 781.674.4200